CCMO, CBG and EMA at Lifesciences@work Expert Class!
It was a great pleasure to have welcomed Monique Al, Coordinating/specialist advisor at CCMO, dr. ir. Marjon Pasmooij, Programme manager Science at CBG and last but not least Ralf Herold MD PhD, Senior scientific officer at EMA at our expert class. The room was filled with startup companies wanting to learn about how to interact with these regulatory bodies. Many thanks to our sponsor and host of the day Loyens and Loeff.
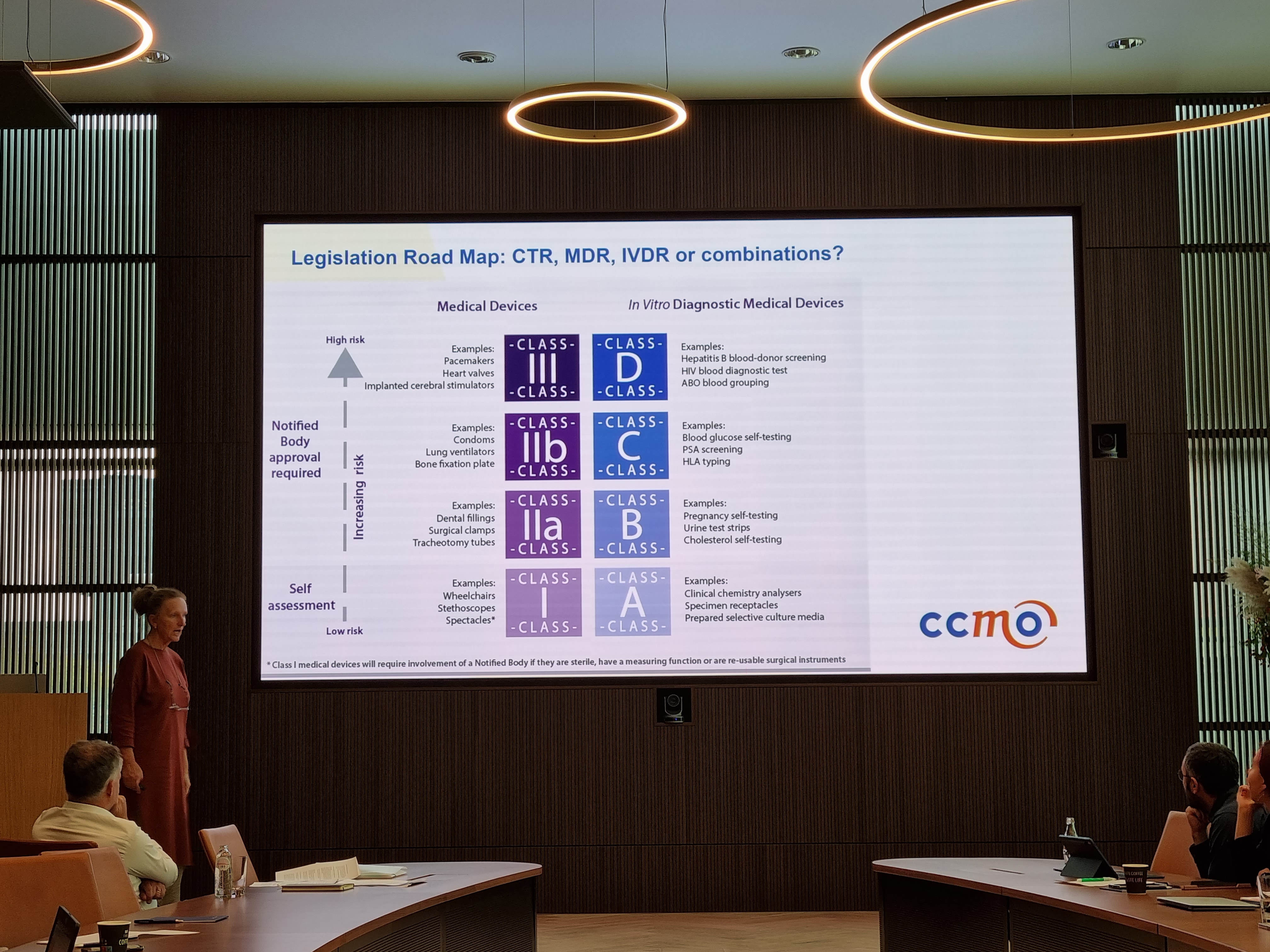
For many LifeSciences startups it is difficult to determine when to contact what regulatory body. Often it is hard to determine in what class an innovative product falls. Is my product a medicinal product or a medical device - or a combination of both? Some startups got clear answers to their question, for others it wasn't as clear cut.
What stood out was that at first the CCMO, CBG and EMA weren't seen as approachable by the audience. During the expert class it became clear that especially the EMA is working hard to change this. For example, did you know the EMA has an SME office, dedicated to help SMEs developing new medicines? In the link you will find ways to contact the EMA for advice. The most important message of the day:
Seek advice as early as possible. If you're too early, you will learn what would be a good time, but at least you won't be too late. - Ralf Herold, EMA
An impression of the afternoon: